Generic Drug CRO Market Size to Climb to USD 11.73 Billion by 2034, reports Towards Healthcare
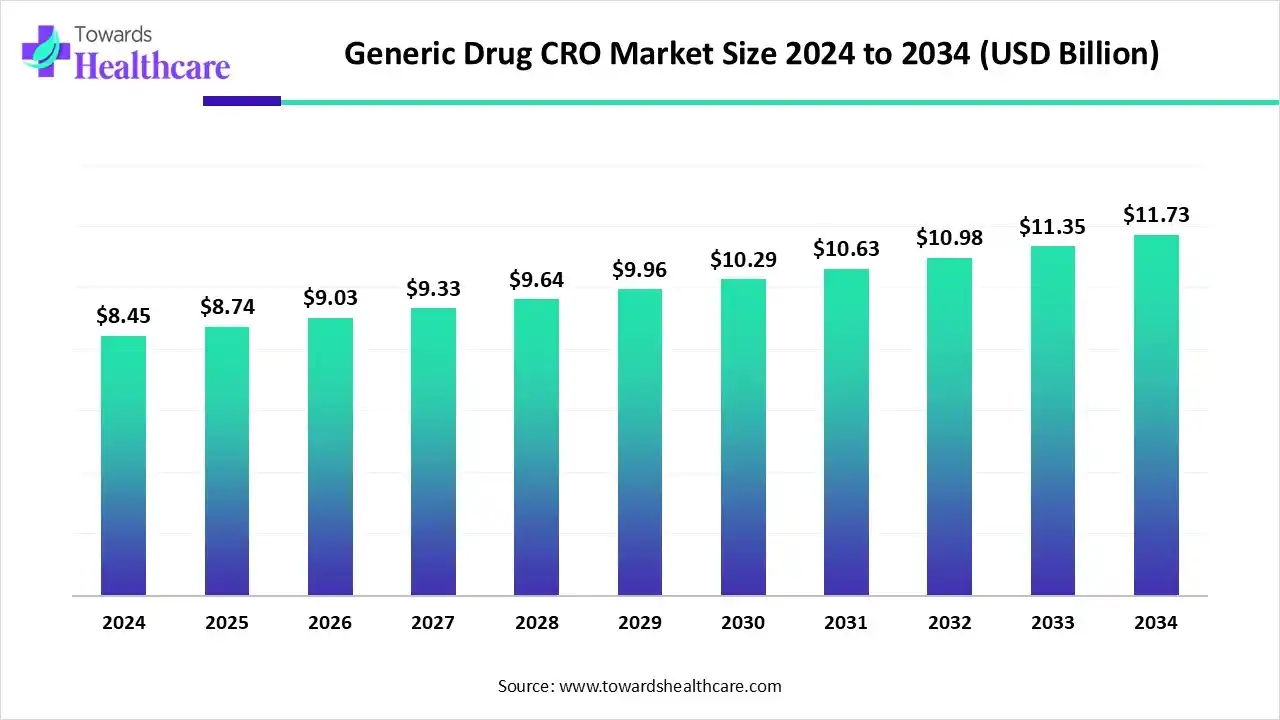
The global generic drug CRO market size was valued at USD 8.45 billion in 2024 and is predicted to hit around USD 11.73 billion by 2034, rising at a 3.36% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
Ottawa, Nov. 25, 2025 (GLOBE NEWSWIRE) -- The global generic drug CRO market size is calculated at USD 8.74 billion in 2025 and is expected to reach around USD 11.73 billion by 2034, growing at a CAGR of 3.36% for the forecasted period.
The generic drug CRO market is rising due to accelerating demand for cost-efficient clinical development solutions, increasing generic drug approvals, and expanding outsourcing trends across global pharmaceutical industries.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/6335
Key Takeaways:
- The generic drug CRO market will likely exceed USD 8.45 billion by 2024.
- Valuation is projected to hit USD 11.73 billion by 2034.
- Estimated to grow at a CAGR of 3.36% starting from 2025 to 2034.
- By region, the North America segment was dominant in the generic drug CRO market in 2024, with approximately 40% share.
- By region, the Asia Pacific segment is expected to be the fastest-growing over the forecast period, 2025 to 2034.
- By service type, the clinical development services segment was dominant in 2024, with approximately 45% share.
- By service type, the laboratory services segment is expected to be the fastest-growing over the forecast period, 2025 to 2034.
- By dosage form, the oral solids segment was dominant in the generic drug CRO market in 2024, with approximately 50% share.
- By dosage form, the injectable formulations segment is expected to be the fastest-growing over the forecast period, 2025 to 2034.
- By end user, the generic pharmaceutical companies segment was dominant in 2024, with approximately 50% share.
- By end user, the contract manufacturing organizations (CMOs) segment is expected to be the fastest-growing over the forecast period, 2025 to 2034.
- By business model, the full-service CROs segment was dominant in 2024, with approximately 45% share.
- By business model, the specialty/niche CROs segment is expected to be the fastest-growing over the forecast period, 2025 to 2034.
- By technology/platform adoption, the analytical & bioanalytical platforms segment was dominant in the generic drug CRO market in 2024, with approximately 35% share.
- By technology/platform adoption, the cloud-based data management & analytics segment is expected to be the fastest-growing over the forecast period, 2025 to 2034.
Market Overview:
What is contributing to the rapid growth of the global generic drug CRO market?
The global generic drug CRO (contract research organization) market is rapidly changing as pharmaceutical companies continue to use outsourced research services to reduce development costs and speed along regulatory approvals. Other factors such as increased price sensitivity in healthcare, rising patent expiries, as well as a generally greater need for specialized support in bioequivalence studies are broadening CRO engagements in the world.
In addition, the rapid growth of generic drug production in emerging economies increases the demand for integrated CRO solutions that meet clinical trial needs, laboratory requirements, formulation development, and regulatory guidance. As regulatory pathways become more complicated and require additional clinical expectations, generic drug developers will drive the collaboration with contract research organizations that offer value in faster timelines, more efficient operating models, and global development networks.
Quick Facts Table
| Table | Scope | |
| Market Size in 2025 | USD 8.74 Billion | |
| Projected Market Size in 2034 | USD 11.73 Billion | |
| CAGR (2025 - 2034) | 3.36 | % |
| Leading Region | North America by 40% | |
| Market Segmentation | By Service Type, By Dosage Form, By End-User, By Business Model, By Technology / Platform Adoption, By Region | |
| Top Key Players | Parexel International, ICON plc, Syneos Health, IQVIA, Covance (Labcorp), PPD (Thermo Fisher), Medpace, WuXi AppTec, Eurofins Scientific, Pharmaron, Bioclinica, Frontage Laboratories, SGS Life Sciences, Celerion, Kendle International, Charles River Laboratories, Pharmaceutical Product Development (PPD), CTI Clinical Trial & Consulting Services, Labcorp Drug Development, Nucleus Network | |
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Major Growth Drivers:
What Are the Main Contributors to the Growth of the Generic Drug CRO Market?
- The surge in generic drug approvals globally has led to an increased need for outsourcing so that pharmaceutical companies can work with partners to speed up trial readiness, manage a bioequivalence study, and reduce internal operational capacity. CROs providing integrated regulatory and clinical development services are thriving in this model.
- The increased pressure from ever-increasing healthcare costs is driving the demand for affordable generic drugs and subsequently increases the volume of clinical work and analytical work these products require before they can be launched into a generic market. CROs offer a scalable, efficient mechanism to address the additional workload.
- Patent expirations in major therapeutic classes present possibilities for CROs to offer manufacturing processes to generic drug manufacturers to enable rapid development and commercialization of medicines. Their knowledge of regulatory and compliance processes facilitates these timelines and minimizes delays.
- The increasing complexity of clinical work in the development of generic drugs leads specialty CROs to continue to be part of the solutions as injectables, complicated formulations, and biological generics gain adoption, and increasing laboratory and analytical platform use.
Key Drifts:
What Strategic Changes in Market Structure Are Changing the Regulatory Environment for Generic Drug CROs?
Key changes in market structure consist of greater adoption of digital applications, increased AI-enabled analytics, and more management of trials through cloud-based applications. Generic drug developers see CROs as more valuable when they can offer data-fidelity, speed with the trial process and global regulatory harmonization. We also note a gradual shift toward full-service outsourcing models for CROs that can provide a comprehensive approach for all project needs, whether it be formulation through to post-market surveillance. In parallel, we are seeing an increased demand for specialized niche CROs as drug developers pursue complex generics, sterile injectables and formulations that are biosimilar-equivalents, usually associated with more substantial laboratory infrastructures for their complex chemistries and biological characteristics.
Major Investment by CRO in Generic Drug in 2025
| Company | Investment |
| Dash Bio | In July 2025, Dash Bio Secures $11M to accelerate a new era in bioanalysis, bringing total funding to $17.5M. |
| Avandra | In February 2025, Avandra Launches with $17.75 in Funding to advance the Use of Real-World Medical Data. |
| Incite Health | In May 2025, Incite Health raised a total of $331K from 1 Seed round |
Significant Challenge:
The generic drug CRO market still has to address one of the key challenges, and that is just dealing with the complexities and evolvement of regulations across global markets. For example, there are differences in the requirements for bioequivalence testing, pharmacokinetics and documentation required for clinical trials by the different regulatory authorities, leading to delays in project timelines. It is the ongoing need for CROs to keep up with the continual regulatory upgrades for compliance, audits specific to an area, and local understanding of regulations that results in ethical violations related to the timeline for approval. The variability in regulation increases operational costs and continues to challenge CROs both direct and third-party, regardless of whether long established or soon to be operational.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Regional Analysis:

Due to the highly developed regulatory landscape, the established CRO ecosystem, and a large concentration of generic drug manufacturers in the region, North America continues to dominate the generic drug CRO market. The stringent controls from the FDA and the emphasis on high-quality bioequivalence and clinical testing services are significant factors when considering the U.S. and Canada.
There is an abundance of both large and small CROs based in the U.S.; moreover, large CROs within the region provide an extremely competitive and innovation-driven market. Furthermore, the increased filings in Paragraph IV and the continued expiration of patent drugs has promoted continuous outsourcing of generics. Additionally, advanced laboratories, digital trial platforms, and a scientific workforce capably tailor the services to spur more growth in North America.
Asia-Pacific is the fastest-growing regional market for generic drug CROs as a result of expanding pharmaceutical manufacturing capability, lower costs of operational support, and the rapidly evolving regulatory infrastructure. Countries such as China, India, and South Korea are establishing themselves as global leaders in low-cost clinical studies and large bioequivalence studies. Because of the availability of patients for clinical trials, the region of Asia-Pacific is attractive to multi-national companies looking for an expedited study timeline. In addition, government devices to support generic drug manufacturing and outsourcing of clinical work are accelerating generic drug CRO growth in the region.
Segmental Insights:
By Service Type:
As the largest segment in the generic drug CRO industry in 2024, clinical development services are expected to continue growing due to the growing need for bioequivalence studies, pharmacokinetics evaluation, and multi-center studies. Generic pharmaceutical companies increasingly rely on CROs in the research process to navigate through the complicated clinical processes and expedite their product introduction timeline. The segment benefits from existing networks of sites, site monitoring, and standardized regulatory documentation process
Laboratory services are projected to be the fastest-growing segment from 2025 to 2034, as generic drug developers increasingly rely on CRO laboratories to conduct bioanalytical testing, stability studies, dissolution profiling, and/or impurity testing. Continuing to advance the speed in which clinical development is conducted are the increase in highly technical products, complex injectables, and specialty generics all which are increasing the need for advanced lab platforms. CROs with cutting-edge analytical capabilities with automated functionality are already experiencing overcoming objections.
By Dosage Form:
Oral solids accounted for the largest share in 2024, as the world continues to use significant amounts of tablets and capsules still the most common dosage forms for generic medications. Contract research organizations (CROs) are vital support for formulation optimization, dissolution testing, and bioequivalence studies of oral therapeutics. The demand for low-cost oral generics treatment in the cardiovascular, metabolic, and infectious disease segments continues to continue to solidify the segment’s leadership position. As numerous generic oral therapeutics can be developed through common interlocutor pathways and with less complexity in manufacturing versus injectables, oral solids will maintain pre-eminence in the CRO domain.
Drug product injectable formulations are expected to become the fastest growing segment from 2025 - 2034, as complex injectables, sterile formulations, and long-acting generics are becoming more popular in hospitals. Contract research organizations (CROs) frequently partner with manufacturers to manage specialized drug testing, sterility validation, and stability studies for injectable compounds. As the transfer to drug administration in hospitals expands within the global professional landscape, demand for harmonized, quality contract research organization (CROs) support for drug product development and analytic testing will continue to intensify.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By End User:
Generic pharmaceutical companies were the largest market share segment by end-user category in 2024 since they leverage CROs for formulation development, clinical trial execution, regulatory submission and approval, and post-approval studies. In saturated therapeutic areas, to expedite the development timeline and gain a competitive edge, generic drug manufacturers utilize outsourced services heavily. In response to increasing pricing pressure from global healthcare systems, generic drug manufacturers are turning to CROs that offer services at lower cost levels than previously available.
The segment representing Contract Manufacturing Organizations is projected to be the fastest- growing end-user segment based on their evolving role in integrated "development to manufacturing" service model. As CMOs scale capabilities for sterile injectables, advanced formulations, and high-volume production, they combine their efforts with increasing CRO for analytical testing, clinical documentation, and regulatory compliance documentation. This trend increases the value of a broader development and reporting (D&R) through the end-to-end outsourcing model.
By Business Model:
Full-Service CROs continued to dominate the market in 2024 as demand for comprehensive services continues to increase, which includes assortment services ranging from early formulation, clinical development, regulatory affairs, and post-approval studies. Generic drug developers are drawn to full-service CRO partnerships to fast-track time to market and ensure uniform regulatory compliance across the globe. These CROs offer broad capabilities such as integrated platforms, and clinical networks, and laboratory services, making it easy to take care of the added complexity of multi-vendor management when conducting studies.
Specialty and niche CROs are expected to be the fastest-growing segment between 2025 to 2034 given the expected increased demand for capabilities that address the complexity of generics, injectables, sterile formulations, advanced analytical services, or other complicated therapeutic areas. These CROs provide highly specialized capabilities that are critical for solving both scientific and regulatory issues associated with emerging generics. Their speed, innovation-centric methodologies, and specialized centered services resonate with pharmaceutical companies looking for both precision and technical depth.
By Technology/Platform Adoption:
In 2024, analytical and bioanalytical platforms won't just represent the largest segment of FDA submissions but will be strongly influenced by the immense demand for accurate analyses, impurity characterization, stability studies, and bioequivalence measures. CROs with well-equipped analytical infrastructures and instrumentation play a vital role in regulatory compliance and quality oversight at the generic drug development and submission stage. As drug formulations become more complex than ever, the demand for reliable and high-accuracy analysis will be even more compelling.
Cloud-based data management and analytics platforms are expected to be the fastest-growing segment in the 2025-2034 outlook period as digital transformations in use of cloud technology and data management accelerates in clinical research. Not only are CROs adopting cloud ecosystems to simplify trial monitoring and allow for real-time data-shared environments with regulatory documentation, the adoption of AI analytics alone will enhance trial performance and the quality of decision-making throughout the trial life cycle. As more generic drug developers embrace digital transparency and speed, cloud-based CRO services are expected to grow at a significant pace throughout the forecast period.
Browse More Insights of Towards Healthcare:
The generic pharmaceuticals market was estimated at US$ 392.23 billion in 2023 and is projected to grow to US$ 947.67 billion by 2034, rising at a compound annual growth rate (CAGR) of 8.35% from 2024 to 2034.
The global generic pharmaceuticals contract manufacturing market size is calculated at US$ 81.24 billion in 2025, grew to US$ 85.99 billion in 2026, and is projected to reach around US$ 143.22 billion by 2035. The market is expanding at a CAGR of 5.85% between 2026 and 2035.
The global generic sterile injectable market was estimated at US$ 42.42 billion in 2023 and is projected to grow to US$ 119.82 billion by 2034, rising at a compound annual growth rate (CAGR) of 9.9% from 2024 to 2034.
The global inhalation and nasal spray generic drugs market size was US$ 25.18 billion in 2025, grew to US$ 27.44 billion in 2026, and is projected to reach around US$ 59.35 billion by 2035. The market is expected to expand at a CAGR of 8.99% between 2026 and 2035.
The global branded generics market size is calculated at US$ 383.1 billion in 2025, grew to US$ 415.54 billion in 2026, and is projected to reach around US$ 867.21 billion by 2035. The market is expanding at a CAGR of 8.47% between 2026 and 2035.
Recent Developments:
- In March 2024, IQVIA announced the launch of its advanced Bioequivalence Accelerator Platform, designed to shorten BE study timelines using AI-enabled analytics and adaptive trial workflows. This platform aims to enhance data accuracy and improve regulatory submission efficiency.
Generic Drug CRO Market Key Players List:
- ICON plc
- Syneos Health
- IQVIA
- Covance (Labcorp)
- PPD (Thermo Fisher)
- Medpace
- WuXi AppTec
- Eurofins Scientific
- Pharmaron
- Bioclinica
- Frontage Laboratories
- SGS Life Sciences
- Celerion
- Kendle International
- Charles River Laboratories
- Pharmaceutical Product Development (PPD)
- CTI Clinical Trial & Consulting Services
- Labcorp Drug Development
- Nucleus Network
Segments Covered in the Report
By Service Type
- Preclinical Services
- In Vitro / Analytical Testing
- Formulation Development
- Toxicology / Safety Studies
- Clinical Development Services
- Bioequivalence / Pharmacokinetic Studies
- Clinical Phase I / II Trials
- Comparative Bioavailability Studies
- Regulatory & Submission Services
- ANDA / Regulatory Submissions
- Compliance & Documentation Support
- Laboratory Services
- Analytical & Quality Control Testing
- Stability Studies
- Others
By Dosage Form
- Oral Solids
- Oral Liquids
- Injectable Formulations
- Topical / Transdermal
- Others
By End-User
- Generic Pharmaceutical Companies
- Biotechnology Companies
- Contract Manufacturing Organizations (CMOs)
- Government / Public Health Organizations
By Business Model
- Full-Service CROs
- Specialty / Niche CROs
- Hybrid CROs
By Technology / Platform Adoption
- Bioanalytical & Analytical Platforms
- Electronic Data Capture (EDC) & eClinical Platforms
- Automated Formulation Development Platforms
- Cloud-Based Data Management & Analytics
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/6335
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

